Abstract
Haemophagocytic lymphohistiocytosis (HLH) is a life-threatening systemic inflammatory clinical syndrome that can be primary/familial or secondary to a variety of underlying conditions. The 2004 diagnostic criteria for HLH require 5 out of 8 of the following clinical and pathological variables to be present: fever, splenomegaly, cytopenia in at least 2 lineages, hypertriglyceridemia/hypofibrinogenemia, haemophagocytosis on pathology examination, low/absent NK cell activity, ferritin greater than 500µg/L or soluble IL-2 receptor (sCD25) greater than 2400 U/mL. At least 5 of these criteria must be met, as each one lacks specificity. With regard to the pathology detection of haemophagocytosis, the sensitivity and specificity reported in the literature were 83% and 60% respectively. Indeed, a degree of haemophagocytosis can be seen outside the context of HLH. Furthermore, some of the diagnostic features of HLH may not be useful when applied to single cases because they may be intrinsic to the underlying condition. Therefore, we wondered whether the histopathological diagnosis might be improved. We investigated whether testing for S100B expression in macrophages might render the detection of haemophagocytosis more specific and sensitive. S100B is a marker of macrophage activation, and can routinely be detected by immunohistochemistry. S100B has pro-inflammatory properties and has been shown to affect macrophage function. It is typically expressed in macrophages of Rosai-Dorfman disease apart of having also a tissue-specific expression pattern, marking melanocytes as well as Langerhans cells and other subsets of dendritic cells.
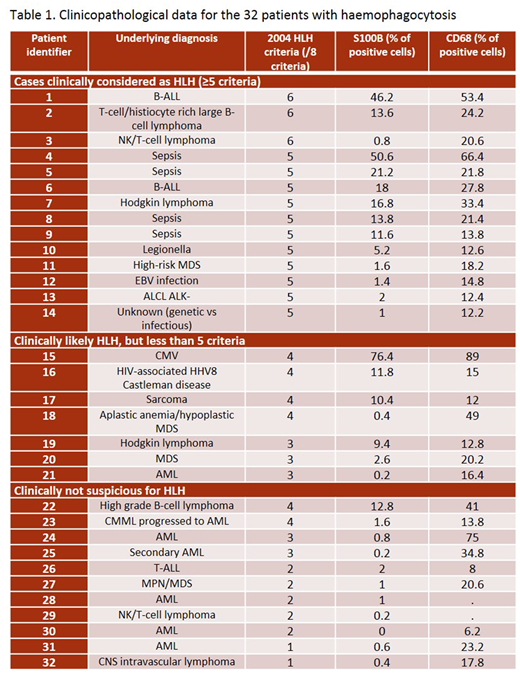
A natural language search of the pathology database at our institution identified 32 patients with bone marrow samples reporting haemophagocytosis as evaluated on bone marrow smear preparations, between January 2002 and July 2015. Cases that did not have available paraffin-embedded bone marrow trephine biopsy and clinical data were excluded. The bone marrow samples of three patients without haemophagocytosis and without any clinical features of HLH were used as controls: a new diagnosis of acute leukemia, a follow-up of acute leukemia post-transplant and a known lymphoma patient with unexplained pancytopenia. Clinical parameters relevant to the diagnosis of HLH were evaluated. Cases were clinically categorized as diagnostic for HLH (≥5 criteria), clinically likely (<5 criteria, but clinical presentation consistent) or clinically non suspicious (<5 criteria and clinical presentation not consistent with HLH). Stains for S100B and CD68 were performed. The percentage of S100B positive cells of all bone marrow cells as well as the percentage of CD68 positive macrophages was calculated from 500-cell counts using a 40x objective lens.
The median patient age was 57 years (range 18-80). Underlying clinical diagnoses are reported in Table 1. Expression of S100B ranged from 0-76.4%. Cases without morphological evidence of haemophagocytosis had less than 1% S100B-positive cells. Cases with presence of haemophagocytic cells showed various levels of S100B-positive cells. Of interest, >10% S100B-positive cells (of all cells) was 100% specific for cases meeting ≥4 HLH criteria, 91% specific for cases clinically likely HLH or confirmed HLH. The sensitivity was 60% for cases meeting ≥4 HLH criteria, 52% for cases being likely HLH or confirmed HLH. Percentage of CD68-positive cells or S100B/CD68 ratio did not add relevant information.
The high specificity of S100B expression by immunohistochemistry indicates it may be an additional diagnostic feature of HLH. Of note, the test is available in routine immunohistochemistry laboratories. Since not all cases of HLH show increased numbers of S100B-expressing macrophages, the test does not obviate the need for the 2004 criteria. Serological immunoassays for S100B have been developed for use in the context of brain disease/injuries and malignant melanoma. Based on our data, we intend to further investigate the sensitivity and specificity of S100B as a serological marker of HLH.
Kuruvilla:BMS: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy; Amgen: Honoraria; Seattle Genetics: Consultancy, Honoraria; Leukemia and Lymphoma Society Canada: Research Funding; Merck: Consultancy, Honoraria; Karyopharm: Honoraria; Celgene: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Princess Margaret Cancer Foundation: Research Funding; Lundbeck: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal